You will need:
- Hot water from a kettle or thermos
CAUTION: adults to use this only!
- A glass bottle
- A balloon
- A tall plastic container


School science visits since 2004!
– Curriculum-linked & award-winning incursions.
– Over 40 primary & high school programs to choose from.
– Designed by experienced educators.
– Over 2 million students reached.
– Face to face incursions & online programs available.
– Early learning centre visits too!
What is going on?
Hot gases expand, cold gases contract!
All gases have freely moving molecules. As you add more heat to a gas, the molecules move faster and push harder against the sides of a vessel that holds them. The more the gas molecules collide against the sides of the vessel, the greater the air pressure.
By covering the glass bottle in hot water, the heat energy moved into the contained air which then increased the air molecules movement inside the bottle and therefore the air pressure as well. This increased air pressure expanded the balloon!
Variable testing
More about variable testing here
- Try different size balloons. Can you inflate each one?
- With an adult, try different water temperatures
- Try different shaped bottles
Going further
What you have observed is an example of how the pressure of a gas is proportional to the temperature of the gas. In simple terms, this means that if you increase the pressure, you increase the temperature. This works both ways, if you increase the temperature, you increase the pressure!
This is Gay Lussac’s Law, stated more precisely as below:
The pressure of a given mass of gas varies directly with the absolute temperature of the gas, when the volume is kept constan
which can be written mathematically as follows:
whereby:
P1 = Initial pressure
T1 = Initial temperature
P2 = Final pressure
T1 = Final temperature
You can explore the effect of changing temperature, volume & pressure in the interactive simulation by the University of Colarado Boulder PhET Interactive Simulations project
Just click on “Ideal” which refers to what happens to an Ideal Gas, which is a pretend gas which has molecules that don’t chemically react with each other. This means that you can see what happens to these molecules as you change the temperature, pressure and volume of the container much more easily.
Hot & Cold Workshop
Years 1 to 6
Maximum 30 students
School workshop (NSW & VIC)
60 or 90 minutes
Online Class Available
Liquid Nitrogen Show
Years K to 6
Maximum 60 students
Science show
45 minutes
Online Class Available
STEM Full Day Accelerator - Primary
Designed from real classroom experiences, this modular day helps you create consistently effective science learning that directly address the new curriculum with easily accessible and cost-effective materials.






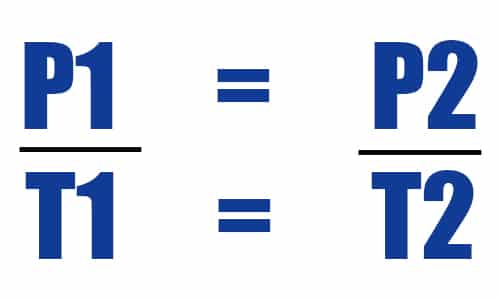

























Comments