You will need:
- Adult Help
- 300g Of Sugar
- Saucepan or kettle with 500mL of water
- Wooden kebab stick or chopstick
- 2 Clothes pegs
- A glass
- A metal spoon
- A place to leave the experiment setup away from ants
- Optional: food colouring


Using pegs to suspend a chopstick on the saturated sugar solution
Use two clothes pegs to suspend the wooden kebab stick or chopstick in the sugar solution without the stick touching the sides of the glass. Place the glass in space where ants cannot get at it (you might want to cover the experiment with a cloth).
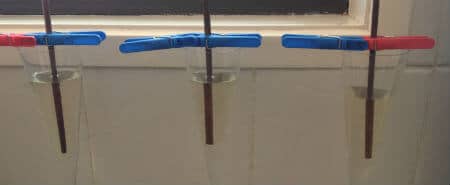
Multiple rock candy experiments
You may want to setup several experiments to see if the crystal formation differs with different amounts of sugar in the water. It’s all about variable testing!

Get the Unit of Work on Mixtures here!
- How can we separate mixtures?
- What are the different techniques?
- From chromatography to magnetism, join us to explore the variety of ways we can separate mixtures!
Includes cross-curricular teaching ideas, student quizzes, a sample marking rubric, scope & sequences & more

School science visits since 2004!
– Curriculum-linked & award-winning incursions.
– Over 40 primary & high school programs to choose from.
– Designed by experienced educators.
– Over 2 million students reached.
– Face to face incursions & online programs available.
– Early learning centre visits too!
Why Does This Happen?
You made a super-saturated solution of sugar and water! The sugar crystals could only stay dissolved whilst the water was hot. Cooling the solution down made it super-saturated, which is unstable. As the water cooled down, less of the sugar crystals could remain in the water and so they began to settle out onto the kebab stick, which effectively acted as a seeding crystal. The sugar was more likely to settle on the kebab stick rather than the glass as the kebab stick has a rougher surface. This rough surface gives plenty of microscopic nucleation points for the molecules of sugar to settle. Over time, more and more of the sugar continued to settle out of the solution onto the kebab stick and so your crystals continued to grow!
Simply put, the longer it takes to form a crystal, the larger the crystal will be.
This works whether you are talking about crystal growing kits, making liquid nitrogen ice-cream or gemstones!
- Check out the link on crystals formed by volcanoes; Indianapolis Childrens Museum
- Find out more about crystal seeding from this Hampton Research paper
Variables to test
- Try starting the experiment with hot vs. cold water… how much of a difference does this make?
- Vary the amount of sugar used in each experiment
- What happens when you use different liquids with sugar dissolved in it?
- Try different substrates for the sugar crystals to cling on too. Which work best?
From crystal growing to slimy science, we’ve got your kitchen chemistry covered!
Get in touch with FizzicsEd to find out how we can work with your class.
Chemistry Show
Years 3 to 6
Maximum 60 students
Science Show (NSW & VIC)
60 minutes
Online Class Available
STEM Full Day Accelerator - Primary
Designed from real classroom experiences, this modular day helps you create consistently effective science learning that directly address the new curriculum with easily accessible and cost-effective materials.































This was pretty helpful. I’m glad this could help me for my science fair project but maybe you guys should include a little bit more on how they actually form and start to clump to the dowel or skewer. Thanks!
Sure thing Amanda! It’s all about how rough the skewers are under a microscope. With super-saturated sugar solutions, the molecules of sugar more easily precipitate onto the rough surfaces of the kebab sticks than the smoother glass container. As the sugar molecules settle on the rough surfaces (also known as nucleation points), the crystallized sugar provides even more rough surfaces for the rest of the sugar to come out of solution. We’ve added a link into the post which takes to a detailed paper on seeding crystals too. Thanks for trying this science experiment, we’re glad that it helped your science fair project!
Does the sugar solution have to be thick for it to work?
Hi Ryan, great question!
I want you to try several versions of thick vs thin (dense vs. less dense)… this way you’re doing real science and not just a trick 🙂
Once you know the answer, pop it in the chat below for everyone and you’ve helped everyone who reads your answer!
but how do you get it dense and less dense?
Love your question Ryan!
Just change the different amount of the sugar syrup that you add to glasses of the same size and then top up with water to the same level. A bit of a mix and you’ve changed the density by diluting the sugar solution for each glass.
How long does it normally take? I’ve had it set up for almost 5 days now and there is little growth. Does it make a difference if we don’t boil the water?
Hi Caitlin!
This has a lot to do with how saturated your solution is as well as how rough the substrate is that the crystals are settling on. Generally, we see crystals from between 1 & 2 weeks however the longer you leave it, the larger the crystals will be. You can try boiling vs not boiling the water too as a fir test, let us know how this goes!
Is it edible ?
Absolutely!
can we use brown sugar with it still working?
Sure thing! Although you’ll find that the rock candy will be darker in colour. Let us know how you go 🙂
So my name is Charlie (Charlotte) how many drops of food colouring do I put in?
Hi Charlie, this is dependent on how dark you would like the rock candy to be. Generally you only need a few drops 🙂
can we use honey if we don’t have 300g of sugar?
We’d love to know how you go!
My name is Lij (Elijah), how many drops do I have to put in then?
Hi Lij, try different drops in different tests and see the difference!
Here’s more about variable testing here