One of the most memorable lab session during my university years was in chemistry when we made mayonnaise. It was part of a module on colloidal suspensions; since mayonnaise is an emulsion. The experiment was so much fun, but I lamented the fact that we couldn’t take it home and eat it because it was not made in a food-safe environment! So today, I’m repeating it in my kitchen!

What you will need
- 2 eggs
- 1 cup of oil
- 1 tablespoon of vinegar/lemon juice
- Electric whisk or beater, or food processor if you have one
- Small bowl
Method
- Separate eggs to get the egg yolks, place in the bowl.

- Start mixing with the electric whisk, adding oil one teaspoon at a time. Whisk for a good 15 seconds before each addition!

- Once the desired consistency has been reached, stir in vinegar. This an important step, because the acidity will help the mayonnaise keep for longer by preventing bacteria from growing too much in the raw egg.

- Add seasoning (Salt, pepper, garlic, wholegrain mustard) if you wish!

What is an emulsion anyway?
It is when two liquids that are normally immiscible or don’t mix, come together where one is dispersed within the other in tiny little bits. This can happen through a lot of vigorous and rapid mixing. The main components in mayonnaise that makes up this emulsion is oil and water (in the form of vinegar). If you’ve done our Layered Liquids or Lava Lamp experiments from the Free Experiments section, you will know that water and oil don’t mix. Just like if we were making a salad dressing with oil and vinegar and it goes cloudy when shaken up, but will eventually settle out into two layers. So how do the components of mayonnaise manage to stay together?
Enter lecithin, an emulsifier present in egg yolks. It is an amphiphilic compound, which means it is hydrophilic and hydrophobic at the same time! Parts of the lecithin molecule are structurally very similar to fats with long carbon chains (highlighted in blue and green in the picture below), but it also contains charged groups (in red) on the other end that is water-loving. This combination of features allows lecithin to bridge the oil droplets and the water it is dispersed in, preventing the oil from gathering into a big clump and keep the emulsion stabilised.
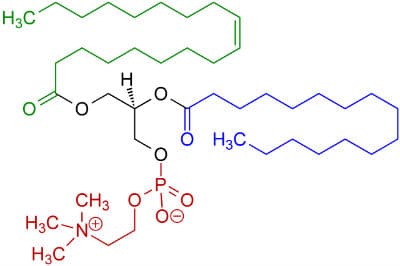
Chemical structure of lecithin
You may notice that your homemade emulsion separates if you add too much oil too quickly, or if each addition was not whisked in enough. Why not use this as an opportunity for some fair-testing and more:
- How much is too much? Record how much oil you can mix in before the emulsion collapses and separates out!
- Does the order matter? Try changing the order in which you do each step and see if it makes a difference. Why might that be?
- What would happen if you used the whole egg? Try it and see!
- Emulsions are delicate systems; what can destroy it? Check out the milk rainbow experiment for more information on this one!
Happy teaching,
Learn more about ‘Marvellous Mixtures’ with the Fizzics team!
NEW Primary science teaching book!
“Be Amazing! How to teach science, the way primary kids love”



























Comments